Introduction: Leukemia is the most common cancer in children and sequencing data suggest that the first genetic alterations often occur in utero. Children with Down syndrome (Trisomy 21, T21) have a 150-fold increased risk of childhood leukemia. In 30% of newborns with Down syndrome, a transient myeloproliferative disorder (pre-leukemia) occurs, which is characterized by a clonal proliferation of immature megakaryoblasts carrying somatic mutations in the GATA1 transcription factor (GATA1s) and resolves spontaneously in most cases. In 20% of the cases, acute megakaryoblastic leukemia (AMKL) evolves from the pre-leukemic clone by acquisition of additional mutations, such as in the cohesin subunit STAG2. It is hypothesized that this represents a multi-step process of leukemogenesis with three distinct genetic events: T21, GATA1s and STAG2. Yet, it remains unclear how an extra copy of chromosome 21 predisposes towards leukemia, the interplay between each genetic event and the cellular origin of transformation.
Methods: Human long-term hematopoietic stem cells (LT-HSCs) were sorted from normal karyotype and T21 fetal livers (N-FL and T21-FL) and subsequently CRISPR/Cas9 edited to try to establish a humanized model of Down Syndrome associated pre-leukemia and AMKL. To model the initiation of the pre-leukemic state, GATA1s mutations were introduced, while additional STAG2- mutations were overlaid to model the progression to fully transformed AMKL. CRISPR/Cas9-edited control, GATA1s, STAG2- and GATA1s/STAG2- LT-HSCs were functionally interrogated in near-clonal xenograft assays, along with transcriptional and epigenetic profiling.
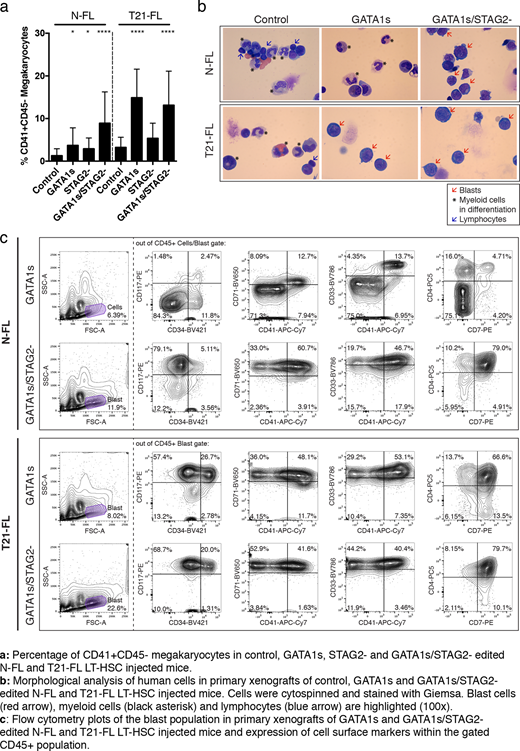
Results: T21 status in combination with GATA1s had a profound synergistic effect on megakaryocytic lineage output in vivo compared to normal karyotype with GATA1s. Moreover, a high percentage of blasts were found in xenografts of GATA1s edited T21-FL LT-HSCs (>30%) but not in xenografts of GATA1s edited N-FL LT-HSCs. Conversely, GATA1s/STAG2- edited LT-HSCs generated grafts with >50% of blasts, regardless of T21 status. The immunophenotype of these blasts recapitulated those observed in patients diagnosed with Down Syndrome pre-leukemia and AMKL (CD117+CD34+CD41+CD71+CD33+CD4+CD7+). Thus, T21 is required for pre-leukemia development, but seems dispensable for AMKL as both N- and T21-FL LT-HSCs underwent leukemic transformation upon GATA1s/STAG2-.
Serial xenotransplantation assays from primary engrafted mice were carried out to assess self-renewal properties of GATA1s-induced pre-leukemia and GATA1s/STAG2- induced AMKL. Only GATA1s/STAG2- edited N- and T21-FL grafts were able to propagate the leukemic phenotype with a high stem cell frequency, which was endowed by the additional STAG2- knock-out. ATACseq and RNAseq profiling of blast populations revealed an enrichment of GATA-binding sites with concomitant up-regulation of genes implicated in translation.
To assess the role of progenitor cells in pre-leukemic initiation and leukemic progression, we CRISPR/Cas9 edited short-term HSCs, common myeloid progenitors and myelo-erythroid progenitors with GATA1s and/or STAG2- and subjected them to xenotransplantation. Strikingly, all progenitor subsets with combined GATA1s/STAG2- editing were able to drive leukemic transformation, while single GATA1s editing in the same subsets did not initiate pre-leukemia. This data strongly suggests that the initial GATA1s mutation must occur in T21 LT-HSCs, but subsequent STAG2 mutations can occur further downstream in progenitors.
Lastly, to gain insight into how chromosome 21 predisposes towards pre-leukemia, three chromosome 21 miRNAs (miR-99a, -125b-2 and -155) were identified to be up-regulated in T21-FL LT-HSCs compared to N-FL LT-HSCs. Over-expression of these miRNAs in N-FL LT-HSCs induced a T21-like state with increased myeloid and megakaryocytic skewing. Dramatically, CRISPR/Cas9-edited knock-out of these miRNAs in GATA1s edited T21-FL LT-HSCs resulted in a block of pre-leukemia initiation.
Conclusion: Our findings demonstrate that T21 is required for pre-leukemia initiation, which is mediated by over-expression of chromosome 21 miRNAs in LT-HSCs. Further, this data demonstrates different cell of origins between pre-leukemia initiation and AMKL progression. Ongoing studies focus on preventing the progression of pre-leukemia to AMKL by pharmacological targeting.
Dick:Bristol-Myers Squibb/Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.